share
GENERAL
What is Full Form of pH? – Potential of Hydrogen Explained
Sept. 7, 2025
614 Views
What is Full Form of pH? – Potential of Hydrogen Explained
Introduction
Many students, professionals, and curious minds often ask: “What is Full Form of pH?”
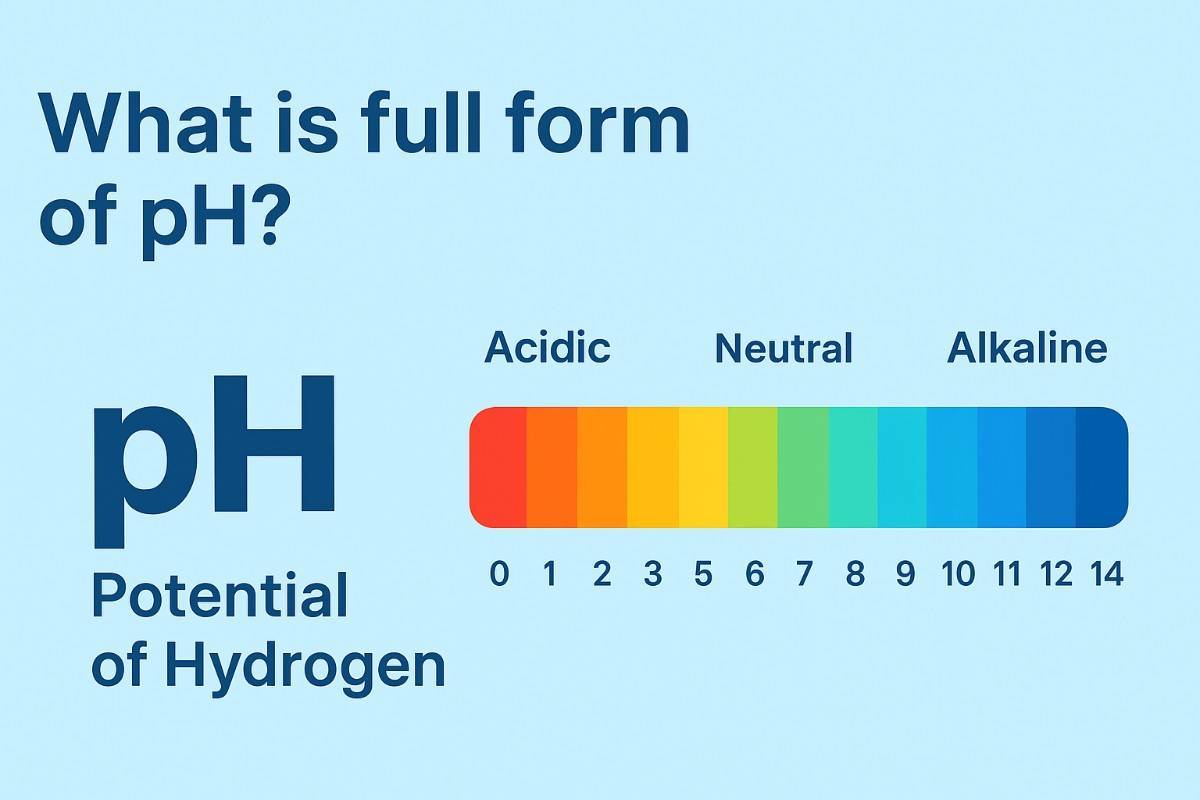
The full form of pH is “Potential of Hydrogen” (sometimes also called Power of Hydrogen).
In simple terms, pH is a scale used in chemistry, biology, medicine, and water science to measure how acidic or alkaline (basic) a solution is. The scale ranges from 0 to 14:
-
pH < 7 → Acidic solution (more hydrogen ions, H⁺)
-
pH = 7 → Neutral solution (pure water)
-
pH > 7 → Alkaline or basic solution (more hydroxide ions, OH⁻)
So whenever you hear pH full form, remember it’s about the Power of Hydrogen, and it tells us how acidic or basic a substance is.
This article covers pH meaning, history, measurement methods, importance in daily life, applications, and FAQs, while targeting important keywords such as:
And more…
Origin and History of pH
If you are wondering, “What is full form of pH in science?”, here’s the detailed background.
The concept of pH was first introduced in 1909 by Søren Peder Lauritz Sørensen, a Danish chemist working at the Carlsberg Laboratory. He wanted a simpler way to express hydrogen ion concentration in solutions.
So, pH stands for Potential (or Power) of Hydrogen.
This simple yet powerful concept became one of the most widely used scales in chemistry, biology, medicine, agriculture, water treatment, and industries worldwide.
How is pH Measured?
Measuring pH levels can be done in different ways depending on accuracy requirements.
| Method |
Description |
Accuracy |
Example Use |
| pH Paper (Litmus Paper) |
Changes color when dipped in solution. The color is compared against a pH scale. |
Low |
Quick classroom or field testing |
| pH Meter |
Electronic device with electrodes that measure hydrogen ion activity. |
Very High |
Laboratories, water treatment plants |
| Indicators |
Special chemicals like phenolphthalein or methyl orange that change color in acidic/basic solutions. |
Medium |
Titration experiments in labs |
For practical water-related pH insights, also read:
The pH Scale – Quick Reference
The pH scale ranges from 0 to 14, and every unit represents a tenfold difference in hydrogen ion concentration.
| pH Value |
Nature |
Examples |
| 0 – 3 |
Strong Acid |
Battery acid, gastric juice |
| 4 – 6 |
Weak Acid |
Tomato juice, vinegar, coffee |
| 7 |
Neutral |
Pure water |
| 8 – 10 |
Weak Base |
Baking soda, seawater |
| 11 – 14 |
Strong Base |
Soap, bleach, cleaning detergents |
So if someone asks: “What is normal pH level?” – the answer depends on the context. For example:
Importance of pH in Daily Life
pH is not just a chemistry concept – it affects our health, environment, food, water, and industries. Let’s see how:
-
Agriculture
-
Water Treatment
-
Medicine & Health
-
Blood pH must remain between 7.35–7.45.
-
A small change can cause serious health issues.
-
Many diseases are linked with abnormal pH levels.
-
Food Industry
-
pH helps control flavor, fermentation, and preservation.
-
Example: Yogurt and pickles rely on acidic pH.
-
Aquariums & Aquatic Life
Applications of pH
pH has applications across multiple industries and sciences:
-
Chemistry & Laboratory Work – Measuring acidity/alkalinity in reactions.
-
Environmental Science – Monitoring acid rain and pollution effects.
-
Medical Field – pH of blood, urine, and body fluids is used in diagnosis.
-
Cosmetics Industry – Shampoos, soaps, and creams are designed to be pH-balanced for skin safety.
-
Agriculture & Soil Testing – Helps farmers improve soil health.
-
Water Purification – Adjusting pH makes water safe for drinking.
For water-related studies, check: Is 25 TDS Safe for Drinking Water?
pH in Different Contexts – Full Forms
| Field |
pH Full Form |
Explanation |
| Chemistry |
Potential of Hydrogen |
Measures acidity/alkalinity of solutions. |
| Biology |
Potential of Hydrogen |
Used in cellular processes and biological fluids. |
| Blood |
Potential of Hydrogen |
Normal blood pH is 7.35–7.45. |
| Human Body |
Potential of Hydrogen |
Vital for homeostasis. |
| Medical |
Potential of Hydrogen |
Used in diagnosis & treatment. |
| Water |
Potential of Hydrogen |
Safe drinking water must have balanced pH. |
| Company/Business |
Potential of Hydrogen |
Sometimes used in product branding. |
FAQs About pH
Q1: What is Full Form of pH?
Ans: The full form of pH is Potential of Hydrogen.
Q2: What do you mean by pH?
Ans: pH is a scale that measures how acidic or basic a solution is.
Q3: Who discovered pH?
Ans: Søren Peder Lauritz Sørensen in 1909.
Q4: What is the pH of pure water?
Ans: Pure water has a neutral pH of 7.
Q5: What is normal pH level?
Ans: It depends:
Q6: What is the full form of pH in NEET exams?
Ans: In NEET (biology/chemistry context), pH means Potential of Hydrogen.
Q7: pH full form in blood?
Ans: pH in blood refers to Potential of Hydrogen, showing the balance of acids and bases in blood.
Q8: pH full form in human body?
Ans: pH helps maintain the acid-base balance in the human body.
Q9: pH definition in simple words?
Ans: pH is the measurement of hydrogen ion concentration in a solution, showing if it’s acidic, neutral, or alkaline.
Related Reads
Conclusion
The full form of pH – Potential of Hydrogen – is a basic yet highly important scientific concept. From soil and water testing to blood health, medicine, and industries, pH is everywhere.
So next time someone asks: “What is Full Form of pH?”, you can confidently say:
-
It means Potential of Hydrogen,
-
It measures how acidic or alkaline a solution is,
-
And it is a key factor in science, health, water, and environment.
share Share now